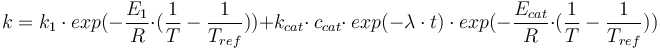Difference between revisions of "Diels-Alder Reaction Experimental Design"
From mintOC
(→Model Formulation) |
(→Model Formulation) |
||
| Line 19: | Line 19: | ||
<math> | <math> | ||
| − | k = k_1 \ \cdot \ exp(- \frac{E_1}{R} \cdot (\frac{1}{T} \ - \ \frac{1}{T_{ref}}) ) + k_{cat} \cdot \ c_{cat} \cdot \ exp(-\lambda \ cdot \ t) | + | k = k_1 \ \cdot \ exp(- \frac{E_1}{R} \cdot (\frac{1}{T} \ - \ \frac{1}{T_{ref}}) ) + k_{cat} \cdot \ c_{cat} \cdot \ exp(-\lambda \ \cdot \ t) \ \cdot \ exp( - \frac{E_{cat}}{R} \cdot (\frac{1}{T} \ - \ \frac{1}{T_{ref}}) ) |
</math> | </math> | ||
Revision as of 10:44, 4 December 2015
This page can now be filled with content.
Model Formulation
Differential equation system:
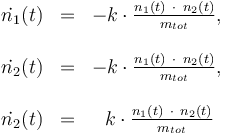
Reaction velocity constant: