Difference between revisions of "Diels-Alder Reaction Experimental Design"
From mintOC
(→Model Formulation) |
(→Model Formulation) |
||
| Line 13: | Line 13: | ||
& & \\ | & & \\ | ||
\dot{n_2}(t) &=& \ \ k \cdot \frac{n_1(t) \ \cdot \ n_2(t)}{m_{tot}} | \dot{n_2}(t) &=& \ \ k \cdot \frac{n_1(t) \ \cdot \ n_2(t)}{m_{tot}} | ||
| + | \end{array} | ||
| + | </math> | ||
| + | |||
| + | The ODE system is summarized to: | ||
| + | |||
| + | <math> | ||
| + | \begin{array}{rcl} | ||
| + | \dot{x}(t) &=& f(x(t), u(t), p) | ||
\end{array} | \end{array} | ||
</math> | </math> | ||
| Line 33: | Line 41: | ||
T(t) = \vartheta (t) + 273 | T(t) = \vartheta (t) + 273 | ||
</math> | </math> | ||
| − | |||
== Optimum Experimental Design Problem == | == Optimum Experimental Design Problem == | ||
Revision as of 13:00, 4 December 2015
This page can now be filled with content.
Model Formulation
Differential equation system:
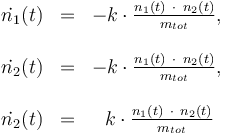
The ODE system is summarized to:
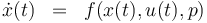
Reaction velocity constant:
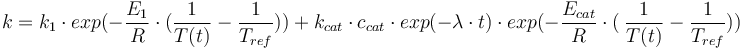
Total mass:
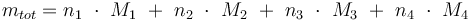
Temperature in Kelvin:
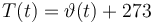
Optimum Experimental Design Problem
![\begin{array}{cll}
\displaystyle \min_{x, G, F, u} && trace(F^{-1} (t_{t_f})) \\[1.5ex]
\mbox{s.t.} \\
\dot{n_1}(t) & = & -k \cdot \frac{n_1(t) \ \cdot \ n_2(t)}{m_{tot}}, \\
\\
\dot{n_2}(t) & = & -k \cdot \frac{n_1(t) \ \cdot \ n_2(t)}{m_{tot}}, \\
\\
\dot{n_2}(t) & = & \ \ k \cdot \frac{n_1(t) \ \cdot \ n_2(t)}{m_{tot}}, \\
\\
\dot{h}(t) & = & \frac{n_3(t) \ \cdot \ M_3}{m_{tot}} \ \cdot \ 100 \\
\\
0 & = & g(x(t_o),x(t_f),p) \\
0 & \ge & c(x,u,p), \forall \, t \in I\\
0 & = & h(x,u,p), \forall \, t \in I \\
x & \in & \mathcal{X},\,u \in \mathcal{U},\, p \in P.
\end{array}](https://mintoc.de/images/math/8/b/7/8b72d12fb7f371499505b8f17e5e612f.png)
| Name | Symbol | Initial value ( ) )
|
| Molar number 1 | 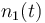
|
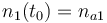
|
| Molar number 2 | 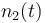
|
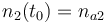
|
| Molar number 3 | 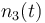
|
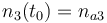
|
| Name | Symbol | Value |
| Molar Mass | 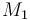
|
0.1362 |
| Molar Mass | 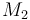
|
0.09806 |
| Molar Mass | 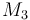
|
0.23426 |
| Molar Mass | 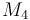
|
0.236 |
| Universal gas constant | 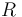
|
8.314 |
| Reference temperature | 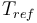
|
293 |
| Name | Symbol | Value |
| Steric factor | 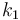
|
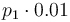
|
| Steric factor | 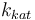
|
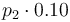
|
| Activation energie | 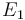
|
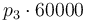
|
| Activation energie | 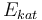
|
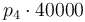
|
| Catalyst deactivation coefficient | 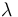
|
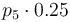
|
with 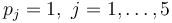
| Name | Symbol | Interval |
| Initial molar number 1 | 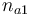
|
[0.4,9.0] |
| Initial molar number 2 | 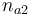
|
[0.4,9.0] |
| Initial molar number 3 | 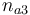
|
[0.4,9.0] |
| Concentration of the catalyst | 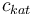
|
[0.0,6.0] |
| Initial molar number 1 | 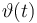
|
[20.0,100.0] |