Difference between revisions of "Diels-Alder Reaction Experimental Design"
From mintOC
| Line 17: | Line 17: | ||
\dot{n_3}(t) &=& \ \ k \cdot \frac{n_1(t) \ \cdot \ n_2(t)}{m_{tot}} \\ | \dot{n_3}(t) &=& \ \ k \cdot \frac{n_1(t) \ \cdot \ n_2(t)}{m_{tot}} \\ | ||
\dot{n_4}(t) &=& 0 | \dot{n_4}(t) &=& 0 | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
\end{array} | \end{array} | ||
</math> | </math> | ||
| Line 108: | Line 100: | ||
|<math>n_3(t)</math> | |<math>n_3(t)</math> | ||
|<math>n_3(t_0) = 0 </math> | |<math>n_3(t_0) = 0 </math> | ||
| + | |- | ||
| + | |Solvent | ||
| + | |<math>n_4(t)</math> | ||
| + | |<math>n_4(t_0) = n_{a4} </math> | ||
|} | |} | ||
| + | |||
{| class="wikitable" | {| class="wikitable" | ||
Revision as of 15:48, 8 December 2015
The Diels-Alder Reaction is an organic chemical reaction. A conjugated diene and a substituted alkene react and form a substituted cyclohexene system.
More information about the reaction can be found in ...
Model Formulation
Differential equation system:
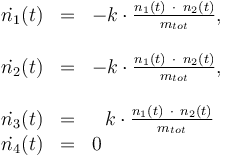
Reaction velocity constant:
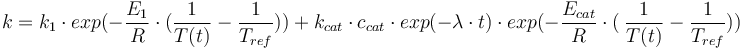
Total mass:
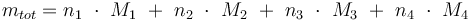
Temperature in Kelvin:
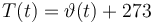
The ODE system is summarized to:
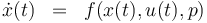
Optimum Experimental Design Problem
![\begin{array}{cll}
\displaystyle \min_{x, G, F, u} && trace(F^{-1} (t_{end})) \\[1.5ex]
\mbox{s.t.} \\
\dot{x}(t) & = & f(x(t), u(t),p), \\
\\
\dot{h}(t) & = & \frac{n_3(t) \ \cdot \ M_3}{m_{tot}} \ \cdot \ 100 \\
\\
\dot{G}(t) & = & f_x(x(t),u(t),p)G(t) \ + \ f_p(x(t),u(t),p) \\
\\
\dot{F}(t) & = & w(t) (h_x(x(t),u(t),p)G(t))^T (h_x(x(t),u(t),p)G(t)) \\
\\
0.1 & \le & n_{a1} \ \cdot \ M_1 \ + \ n_{a2} \ \cdot \ M_2 \ + \ n_{a4} \ \cdot \ M_4 \\
\\
10 & \ge & n_{a1} \ \cdot \ M_1 \ + \ n_{a2} \ \cdot \ M_2 \ + \ n_{a4} \ \cdot \ M_4 \\
\\
0.1 & \le & \frac{ n_{a1} \ \cdot \ M_1 \ + \ n_{a2} \ \cdot \ M_2 }{ n_{a1} \ \cdot \ M_1 \ + \ n_{a2} \ \cdot \ M_2 \ + \ n_{a4} \ \cdot \ M_4 } \\
\\
0.7 & \ge & \frac{ n_{a1} \ \cdot \ M_1 \ + \ n_{a2} \ \cdot \ M_2 }{ n_{a1} \ \cdot \ M_1 \ + \ n_{a2} \ \cdot \ M_2 \ + \ n_{a4} \ \cdot \ M_4 } \\
\\
0 & = & \vartheta_{lo}, \quad \forall \, t \in [t_0,2] \\
\\
0 & = & \vartheta_{lo} + \frac{t-2}{6} ( \vartheta_{up} - \vartheta_{lo} ) , \quad \forall \, t \in [2,8] \\
\\
0 & = & \vartheta_{up}, \quad \forall \, t \in [8,t_{end}] \\
\\
x & \in & \mathcal{X},\,u \in \mathcal{U},\, p \in P.
\end{array}](https://mintoc.de/images/math/5/7/9/579cc4c299c3f8fd421654ebc8ece2de.png)
| Name | Symbol | Initial value (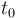 ) )
|
| Molar number 1 | 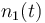
|
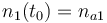
|
| Molar number 2 | 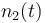
|
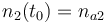
|
| Molar number 3 | 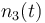
|
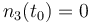
|
| Solvent | 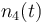
|
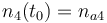
|
| Name | Symbol | Value |
| Molar Mass | 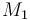
|
0.1362 |
| Molar Mass | 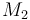
|
0.09806 |
| Molar Mass | 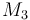
|
0.23426 |
| Molar Mass | 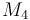
|
0.236 |
| Universal gas constant | 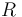
|
8.314 |
| Reference temperature | 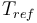
|
293 |
| St.dev of measurement error | 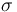
|
1 |
| Name | Symbol | Value |
| Steric factor | 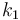
|
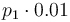
|
| Steric factor | 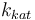
|
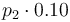
|
| Activation energie | 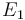
|
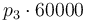
|
| Activation energie | 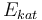
|
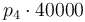
|
| Catalyst deactivation coefficient | 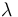
|
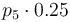
|
with 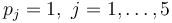
| Name | Symbol | Interval |
| Initial molar number 1 | 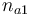
|
[0.4,9.0] |
| Initial molar number 2 | 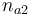
|
[0.4,9.0] |
| Initial molar number 4 | 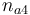
|
[0.4,9.0] |
| Concentration of the catalyst | 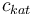
|
[0.0,6.0] |
| Initial molar number 1 | 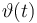
|
[20.0,100.0] |
Measurement grid
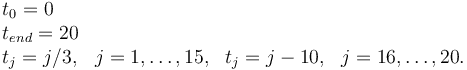
References
R. T. Morrison and R.N. Boyd. Organic Chemistry. Allyn and Bacon, Inc., 4th edition, 1983 \\ Dissertation Stefan Körkel