Difference between revisions of "Diels-Alder Reaction Experimental Design"
(→Model Formulation) |
FelixMueller (Talk | contribs) (→Source Code) |
||
| (138 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | + | The '''Diels-Alder Reaction''' is an organic chemical reaction. | |
| + | A conjugated diene and a substituted alkene react and form a substituted cyclohexene system. | ||
| + | Stefan Körkel used this model in his PhD thesis to compute optimum experimental designs. | ||
== Model Formulation == | == Model Formulation == | ||
| − | + | The reactionkinetics can be modelled by the following differential equation system: | |
<math> | <math> | ||
| Line 12: | Line 14: | ||
\dot{n_2}(t) &=& -k \cdot \frac{n_1(t) \ \cdot \ n_2(t)}{m_{tot}}, \\ | \dot{n_2}(t) &=& -k \cdot \frac{n_1(t) \ \cdot \ n_2(t)}{m_{tot}}, \\ | ||
& & \\ | & & \\ | ||
| − | \dot{ | + | \dot{n_3}(t) &=& \ \ k \cdot \frac{n_1(t) \ \cdot \ n_2(t)}{m_{tot}} \\ |
| + | & & \\ | ||
| + | \dot{n_4}(t) &=& 0 | ||
\end{array} | \end{array} | ||
</math> | </math> | ||
| − | + | The reaction velocity constant <math>k</math> consists of two parts. One part reflects the non-catalytic and the other the catalytic reaction. The velocity law follows the Arrhenius relation | |
| + | |||
<math> | <math> | ||
| − | k = k_1 \cdot | + | k = k_1 \ \cdot \ exp(- \frac{E_1}{R} \ \cdot \ (\frac{1}{T(t)} \ - \ \frac{1}{T_{ref}}) ) \ + \ k_{cat} \ \cdot \ c_{cat} \ \cdot \ exp(-\lambda \ \cdot \ t) \ \cdot \ exp( - \frac{E_{cat}}{R} \ \cdot \ (\ \frac{1}{T(t)} \ - \ \frac{1}{T_{ref}}) ) |
| + | </math> | ||
| + | |||
| + | Total mass: | ||
| + | |||
| + | <math> | ||
| + | m_{tot} = n_1 \ \cdot \ M_1 \ + \ n_2 \ \cdot \ M_2 \ + \ n_3 \ \cdot \ M_3 \ + \ n_4 \ \cdot \ M_4 | ||
| + | </math> | ||
| + | |||
| + | Temperature in Kelvin: | ||
| + | |||
| + | <math> | ||
| + | T(t) = \vartheta (t) + 273 | ||
| + | </math> | ||
| + | |||
| + | The ODE system is summarized to: | ||
| + | |||
| + | <math> | ||
| + | \begin{array}{rcl} | ||
| + | \dot{x}(t) &=& f(x(t), u(t), p) | ||
| + | \end{array} | ||
| + | </math> | ||
| + | |||
| + | == Constraints == | ||
| + | |||
| + | The control variables are constrained with respect to the mass of sample weights (initial mass): | ||
| + | |||
| + | |||
| + | <math> | ||
| + | \begin{array}{cll} | ||
| + | 0.1 & \le & n_{a1} \ \cdot \ M_1 \ + \ n_{a2} \ \cdot \ M_2 \ + \ n_{a4} \ \cdot \ M_4 \le 10 | ||
| + | \end{array} | ||
| + | </math> | ||
| + | |||
| + | and to the mass of active ingredient content (fraction of active substances): | ||
| + | |||
| + | <p> | ||
| + | <math> | ||
| + | \begin{array}{cll} | ||
| + | 0.1 & \le & \frac{ n_{a1} \ \cdot \ M_1 \ + \ n_{a2} \ \cdot \ M_2 }{ n_{a1} \ \cdot \ M_1 \ + \ n_{a2} \ \cdot \ M_2 \ + \ n_{a4} \ \cdot \ M_4 } \le 0.7 | ||
| + | \end{array} | ||
| + | </math> | ||
| + | |||
| + | == Optimum Experimental Design Problem == | ||
| + | |||
| + | The aim is to compute an optimal experimental design <math>\xi = (q,w)</math> which minimizes the uncertainties of the parameters <math>k_1, k_{cat}, E_1, E_{cat}, \lambda</math>. So, we have to solve the following optimum experimental design problem: | ||
| + | |||
| + | |||
| + | <math> | ||
| + | \begin{array}{cll} | ||
| + | \displaystyle \min_{x^i,\ G^i,\ F^i,\ Tc^i,\ n_{a1}^i,\ n_{a2}^i,\ n_{a4}^i,\ c_{kat}^i,\ \vartheta(t)^i} && trace(F^{-1} (t_{end})) \\[1.5ex] | ||
| + | \mbox{s.t.} \\ | ||
| + | \dot{x}^i(t) & = & f(x^i(t), u^i(t),p), \\ | ||
| + | \\ | ||
| + | h(t) & = & \frac{n_3(t) \ \cdot \ M_3}{m_{tot}} \ \cdot \ 100 \\ | ||
| + | \\ | ||
| + | \dot{G}^i(t) & = & f_x(x^i(t),u^i(t),p)G^i(t) \ + \ f_p(x^i(t),u^i(t),p) \\ | ||
| + | \\ | ||
| + | \dot{F}(t) & = & \sum\limits_{i=1}^{4} w^i(t) (h^i_x(x^i(t),u^i(t),p)G^i(t))^T (h^i_x(x^i(t),u(t),p)G^i(t)) \\ | ||
| + | \\ | ||
| + | 0.1 & \le & n_{a1} \ \cdot \ M_1 \ + \ n_{a2} \ \cdot \ M_2 \ + \ n_{a4} \ \cdot \ M_4 \\ | ||
| + | \\ | ||
| + | 10 & \ge & n_{a1} \ \cdot \ M_1 \ + \ n_{a2} \ \cdot \ M_2 \ + \ n_{a4} \ \cdot \ M_4 \\ | ||
| + | \\ | ||
| + | 0.1 & \le & \frac{ n_{a1} \ \cdot \ M_1 \ + \ n_{a2} \ \cdot \ M_2 }{ n_{a1} \ \cdot \ M_1 \ + \ n_{a2} \ \cdot \ M_2 \ + \ n_{a4} \ \cdot \ M_4 } \\ | ||
| + | \\ | ||
| + | 0.7 & \ge & \frac{ n_{a1} \ \cdot \ M_1 \ + \ n_{a2} \ \cdot \ M_2 }{ n_{a1} \ \cdot \ M_1 \ + \ n_{a2} \ \cdot \ M_2 \ + \ n_{a4} \ \cdot \ M_4 } \\ | ||
| + | \\ | ||
| + | \vartheta(t) & = & \left\{ \begin{array}{cl} \vartheta_{lo} + 273 & t \in [t_0,2] \\ | ||
| + | \vartheta_{lo} + \frac{t-2}{6} ( \vartheta_{up} - \vartheta_{lo} ) + 273 & t \in [2,8] \\ | ||
| + | \vartheta_{up} + 273 & t \in [8,t_{end}] | ||
| + | \end{array} \right. \\ | ||
| + | & & x \in \mathcal{X},\,u \in \mathcal{U},\, p \in P \\ | ||
| + | \dot{z}^i(t) & = & w^i(t) \\ | ||
| + | z(0) & = & 0 \\ | ||
| + | w^i(t) &\in& [0,1] \\ | ||
| + | 0 & \le & 4 - z^i(t_f). \\ | ||
| + | \end{array} | ||
| + | </math> | ||
| + | </p> | ||
| + | |||
| + | |||
| + | |||
| + | {| class="wikitable" | ||
| + | |+State variables | ||
| + | |- | ||
| + | |Name | ||
| + | |Symbol | ||
| + | |Initial value (<math>t_0</math>) | ||
| + | |- | ||
| + | |Molar number 1 | ||
| + | |<math>n_1(t)</math> | ||
| + | |<math>n_1(t_0) = n_{a1} </math> | ||
| + | |- | ||
| + | |Molar number 2 | ||
| + | |<math>n_2(t)</math> | ||
| + | |<math>n_2(t_0) = n_{a2} </math> | ||
| + | |- | ||
| + | |Molar number 3 | ||
| + | |<math>n_3(t)</math> | ||
| + | |<math>n_3(t_0) = 0 </math> | ||
| + | |- | ||
| + | |Solvent | ||
| + | |<math>n_4(t)</math> | ||
| + | |<math>n_4(t_0) = n_{a4} </math> | ||
| + | |} | ||
| + | |||
| + | |||
| + | {| class="wikitable" | ||
| + | |+Constants | ||
| + | |- | ||
| + | |Name | ||
| + | |Symbol | ||
| + | |Value | ||
| + | |- | ||
| + | |Molar Mass | ||
| + | |<math>M_1</math> | ||
| + | |0.1362 | ||
| + | |- | ||
| + | |Molar Mass | ||
| + | |<math>M_2</math> | ||
| + | |0.09806 | ||
| + | |- | ||
| + | |Molar Mass | ||
| + | |<math>M_3</math> | ||
| + | |0.23426 | ||
| + | |- | ||
| + | |Molar Mass | ||
| + | |<math>M_4</math> | ||
| + | |0.236 | ||
| + | |- | ||
| + | |Universal gas constant | ||
| + | |<math>R</math> | ||
| + | |8.314 | ||
| + | |- | ||
| + | |Reference temperature | ||
| + | |<math>T_{ref}</math> | ||
| + | |293 | ||
| + | |- | ||
| + | |St.dev of measurement error | ||
| + | |<math>\sigma</math> | ||
| + | |1 | ||
| + | |} | ||
| + | |||
| + | Remember, in an optimum experimental design problem the parameters of the model are fixed. But, we minimize the parameter's uncertainties by optimizing over the control variables and functions. | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |+Fixed parameters | ||
| + | |- | ||
| + | |Name | ||
| + | |Symbol | ||
| + | |Value | ||
| + | |- | ||
| + | |Steric factor | ||
| + | |<math>k_1</math> | ||
| + | |<math>p_1 \cdot 0.01</math> | ||
| + | |- | ||
| + | |Steric factor | ||
| + | |<math>k_{kat}</math> | ||
| + | |<math>p_2 \cdot 0.10</math> | ||
| + | |- | ||
| + | |Activation energie | ||
| + | |<math>E_1</math> | ||
| + | |<math>p_3 \cdot 60000</math> | ||
| + | |- | ||
| + | |Activation energie | ||
| + | |<math>E_{kat}</math> | ||
| + | |<math>p_4 \cdot 40000</math> | ||
| + | |- | ||
| + | |Catalyst deactivation coefficient | ||
| + | |<math>\lambda</math> | ||
| + | |<math>p_5 \cdot 0.25</math> | ||
| + | |} | ||
| + | with <math>p_j = 1, \ j =1, \dots, 5</math> | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |+Optimization/control variables | ||
| + | |- | ||
| + | |Name | ||
| + | |Symbol | ||
| + | |Interval | ||
| + | |Initial value Exp 1 | ||
| + | |Initial value Exp 2 | ||
| + | |Initial value Exp 3 | ||
| + | |Initial value Exp 4 | ||
| + | |- | ||
| + | |Initial molar number 1 | ||
| + | |<math>n_{a1}</math> | ||
| + | |[0,10.0] | ||
| + | |1.0 | ||
| + | |1.0 | ||
| + | |1.0 | ||
| + | |1.0 | ||
| + | |- | ||
| + | |Initial molar number 2 | ||
| + | |<math>n_{a2}</math> | ||
| + | |[0,10.0] | ||
| + | |1.0 | ||
| + | |1.0 | ||
| + | |1.0 | ||
| + | |1.0 | ||
| + | |- | ||
| + | |Initial molar number 4 | ||
| + | |<math>n_{a4}</math> | ||
| + | |[0.4,9.0] | ||
| + | |2.0 | ||
| + | |2.0 | ||
| + | |2.0 | ||
| + | |2.0 | ||
| + | |- | ||
| + | |Concentration of the catalyst | ||
| + | |<math>c_{kat}</math> | ||
| + | |[0,10.0] | ||
| + | |0.0 | ||
| + | |1.0 | ||
| + | |2.0 | ||
| + | |3.0 | ||
| + | |} | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |+Control function | ||
| + | |- | ||
| + | |Name | ||
| + | |Symbol | ||
| + | |Time interval | ||
| + | |Value interval | ||
| + | |Initial value Exp 1 | ||
| + | |Initial value Exp 2 | ||
| + | |Initial value Exp 3 | ||
| + | |Initial value Exp 4 | ||
| + | |- | ||
| + | |Initial molar number 1 | ||
| + | |<math>\vartheta(t)</math> | ||
| + | |<math>[t_0,2]</math> | ||
| + | |[20.0,100.0] | ||
| + | |20.0 | ||
| + | |60.0 | ||
| + | |40.0 | ||
| + | |20.0 | ||
| + | |- | ||
| + | |Initial molar number 1 | ||
| + | |<math>\vartheta(t)</math> | ||
| + | |<math>[2,8]</math> | ||
| + | |[20.0,100.0] | ||
| + | |20.0 | ||
| + | |60.0 | ||
| + | |40.0 | ||
| + | |20.0 | ||
| + | |- | ||
| + | |Initial molar number 1 | ||
| + | |<math>\vartheta(t)</math> | ||
| + | |<math>[8,t_{end}]</math> | ||
| + | |[20.0,100.0] | ||
| + | |20.0 | ||
| + | |60.0 | ||
| + | |40.0 | ||
| + | |20.0 | ||
| + | |} | ||
| + | |||
| + | '''Measurement grid''' | ||
| + | |||
| + | <math> | ||
| + | \begin{array}{llll} | ||
| + | t_0 = 0 & & & \\ | ||
| + | t_{end} = 20 & & & \\ | ||
| + | t_j = j/3, & j = 1,\dots, 15, & t_j = j - 10, & j = 16, \dots, 20. | ||
| + | \end{array} | ||
</math> | </math> | ||
| − | == | + | == Source Code == |
| + | * The VPLAN code using [[:Category: VPLAN | VPLAN]] can be found in: [[Diels-Alder Reaction Experimental Design (VPLAN)]] | ||
| + | == References == | ||
| + | R. T. Morrison and R.N. Boyd. Organic Chemistry. Allyn and Bacon, Inc., 4th edition, 1983 | ||
| + | S. Körkel. Numerische Methoden für Optimale Versuchsplanungsprobleme bei nichtlinearen DAE-Modellen.PhD thesis, Universität Heidelberg, Heidelber,2002 | ||
| − | |||
[[Category:Optimum Experimental Design]] | [[Category:Optimum Experimental Design]] | ||
[[Category:ODE model]] | [[Category:ODE model]] | ||
Latest revision as of 15:17, 29 July 2016
The Diels-Alder Reaction is an organic chemical reaction. A conjugated diene and a substituted alkene react and form a substituted cyclohexene system. Stefan Körkel used this model in his PhD thesis to compute optimum experimental designs.
Contents
Model Formulation
The reactionkinetics can be modelled by the following differential equation system:
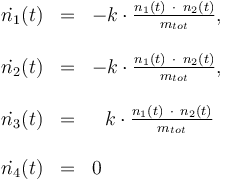
The reaction velocity constant 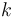 consists of two parts. One part reflects the non-catalytic and the other the catalytic reaction. The velocity law follows the Arrhenius relation
consists of two parts. One part reflects the non-catalytic and the other the catalytic reaction. The velocity law follows the Arrhenius relation
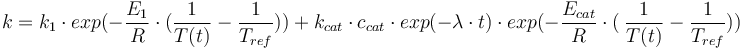
Total mass:
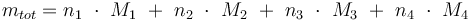
Temperature in Kelvin:
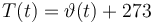
The ODE system is summarized to:
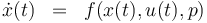
Constraints
The control variables are constrained with respect to the mass of sample weights (initial mass):
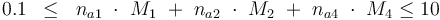
and to the mass of active ingredient content (fraction of active substances):
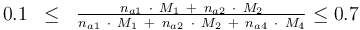
Optimum Experimental Design Problem
The aim is to compute an optimal experimental design 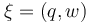 which minimizes the uncertainties of the parameters
which minimizes the uncertainties of the parameters 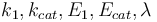 . So, we have to solve the following optimum experimental design problem:
. So, we have to solve the following optimum experimental design problem:
![\begin{array}{cll}
\displaystyle \min_{x^i,\ G^i,\ F^i,\ Tc^i,\ n_{a1}^i,\ n_{a2}^i,\ n_{a4}^i,\ c_{kat}^i,\ \vartheta(t)^i} && trace(F^{-1} (t_{end})) \\[1.5ex]
\mbox{s.t.} \\
\dot{x}^i(t) & = & f(x^i(t), u^i(t),p), \\
\\
h(t) & = & \frac{n_3(t) \ \cdot \ M_3}{m_{tot}} \ \cdot \ 100 \\
\\
\dot{G}^i(t) & = & f_x(x^i(t),u^i(t),p)G^i(t) \ + \ f_p(x^i(t),u^i(t),p) \\
\\
\dot{F}(t) & = & \sum\limits_{i=1}^{4} w^i(t) (h^i_x(x^i(t),u^i(t),p)G^i(t))^T (h^i_x(x^i(t),u(t),p)G^i(t)) \\
\\
0.1 & \le & n_{a1} \ \cdot \ M_1 \ + \ n_{a2} \ \cdot \ M_2 \ + \ n_{a4} \ \cdot \ M_4 \\
\\
10 & \ge & n_{a1} \ \cdot \ M_1 \ + \ n_{a2} \ \cdot \ M_2 \ + \ n_{a4} \ \cdot \ M_4 \\
\\
0.1 & \le & \frac{ n_{a1} \ \cdot \ M_1 \ + \ n_{a2} \ \cdot \ M_2 }{ n_{a1} \ \cdot \ M_1 \ + \ n_{a2} \ \cdot \ M_2 \ + \ n_{a4} \ \cdot \ M_4 } \\
\\
0.7 & \ge & \frac{ n_{a1} \ \cdot \ M_1 \ + \ n_{a2} \ \cdot \ M_2 }{ n_{a1} \ \cdot \ M_1 \ + \ n_{a2} \ \cdot \ M_2 \ + \ n_{a4} \ \cdot \ M_4 } \\
\\
\vartheta(t) & = & \left\{ \begin{array}{cl} \vartheta_{lo} + 273 & t \in [t_0,2] \\
\vartheta_{lo} + \frac{t-2}{6} ( \vartheta_{up} - \vartheta_{lo} ) + 273 & t \in [2,8] \\
\vartheta_{up} + 273 & t \in [8,t_{end}]
\end{array} \right. \\
& & x \in \mathcal{X},\,u \in \mathcal{U},\, p \in P \\
\dot{z}^i(t) & = & w^i(t) \\
z(0) & = & 0 \\
w^i(t) &\in& [0,1] \\
0 & \le & 4 - z^i(t_f). \\
\end{array}](https://mintoc.de/images/math/b/f/d/bfdd6780f8f12eef47eaf2e4a3821ef6.png)
| Name | Symbol | Initial value ( ) )
|
| Molar number 1 | 
|
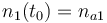
|
| Molar number 2 | 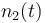
|
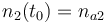
|
| Molar number 3 | 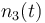
|
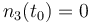
|
| Solvent | 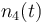
|
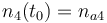
|
| Name | Symbol | Value |
| Molar Mass | 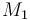
|
0.1362 |
| Molar Mass | 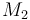
|
0.09806 |
| Molar Mass | 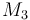
|
0.23426 |
| Molar Mass | 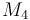
|
0.236 |
| Universal gas constant | 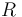
|
8.314 |
| Reference temperature | 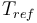
|
293 |
| St.dev of measurement error | 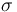
|
1 |
Remember, in an optimum experimental design problem the parameters of the model are fixed. But, we minimize the parameter's uncertainties by optimizing over the control variables and functions.
| Name | Symbol | Value |
| Steric factor | 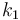
|
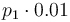
|
| Steric factor | 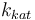
|
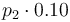
|
| Activation energie | 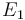
|
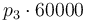
|
| Activation energie | 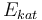
|
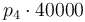
|
| Catalyst deactivation coefficient | 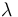
|
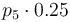
|
with 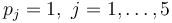
| Name | Symbol | Interval | Initial value Exp 1 | Initial value Exp 2 | Initial value Exp 3 | Initial value Exp 4 |
| Initial molar number 1 | 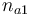
|
[0,10.0] | 1.0 | 1.0 | 1.0 | 1.0 |
| Initial molar number 2 | 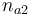
|
[0,10.0] | 1.0 | 1.0 | 1.0 | 1.0 |
| Initial molar number 4 | 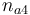
|
[0.4,9.0] | 2.0 | 2.0 | 2.0 | 2.0 |
| Concentration of the catalyst | 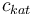
|
[0,10.0] | 0.0 | 1.0 | 2.0 | 3.0 |
| Name | Symbol | Time interval | Value interval | Initial value Exp 1 | Initial value Exp 2 | Initial value Exp 3 | Initial value Exp 4 |
| Initial molar number 1 | 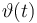
|
![[t_0,2]](https://mintoc.de/images/math/2/c/c/2ccc6e33468338286f8ffb1f62564d08.png)
|
[20.0,100.0] | 20.0 | 60.0 | 40.0 | 20.0 |
| Initial molar number 1 | 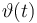
|
![[2,8]](https://mintoc.de/images/math/6/0/f/60fe7d62d10c721e8bc3c5e121a4f922.png)
|
[20.0,100.0] | 20.0 | 60.0 | 40.0 | 20.0 |
| Initial molar number 1 | 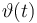
|
![[8,t_{end}]](https://mintoc.de/images/math/c/7/8/c78ee1d499d759d6a276790fc2948860.png)
|
[20.0,100.0] | 20.0 | 60.0 | 40.0 | 20.0 |
Measurement grid
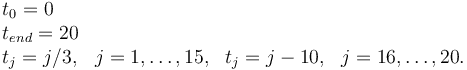
Source Code
- The VPLAN code using VPLAN can be found in: Diels-Alder Reaction Experimental Design (VPLAN)
References
R. T. Morrison and R.N. Boyd. Organic Chemistry. Allyn and Bacon, Inc., 4th edition, 1983 S. Körkel. Numerische Methoden für Optimale Versuchsplanungsprobleme bei nichtlinearen DAE-Modellen.PhD thesis, Universität Heidelberg, Heidelber,2002