Difference between revisions of "Diels-Alder Reaction Experimental Design"
From mintOC
(→Model Formulation) |
(→Model Formulation) |
||
| Line 44: | Line 44: | ||
|Molar number 1 | |Molar number 1 | ||
|<math>n_1(t)</math> | |<math>n_1(t)</math> | ||
| − | |<math>n_1(t_0) = </math> | + | |<math>n_1(t_0) = n_{a1} </math> |
|- | |- | ||
|Molar number 2 | |Molar number 2 | ||
|<math>n_2(t)</math> | |<math>n_2(t)</math> | ||
| − | |<math>n_2(t_0) = </math> | + | |<math>n_2(t_0) = n_{a2} </math> |
|- | |- | ||
|Molar number 3 | |Molar number 3 | ||
|<math>n_3(t)</math> | |<math>n_3(t)</math> | ||
| − | |<math>n_3(t_0) = </math> | + | |<math>n_3(t_0) = n_{a3} </math> |
|} | |} | ||
| Line 85: | Line 85: | ||
{| class="wikitable" | {| class="wikitable" | ||
| − | |+ | + | |+Control variables |
|- | |- | ||
|Name | |Name | ||
|Symbol | |Symbol | ||
|Value | |Value | ||
| − | | | + | |Interval |
|- | |- | ||
| − | | | + | |Initial molar number 1 |
| − | |<math> | + | |<math>n_{a1}</math> |
| − | | | + | |[0.4,9.0] |
| − | |[-] | + | |- |
| + | |Initial molar number 2 | ||
| + | |<math>n_{a2}</math> | ||
| + | |[0.4,9.0] | ||
| + | |- | ||
| + | |Initial molar number 3 | ||
| + | |<math>n_{a3}</math> | ||
| + | |[0.4,9.0] | ||
| + | |- | ||
| + | |Concentration of the catalyst | ||
| + | |<math>c_{kat}</math> | ||
| + | |[0.0,6.0] | ||
| + | |- | ||
| + | |Initial molar number 1 | ||
| + | |<math>\vartheta(t)</math> | ||
| + | |[20.0,100.0] | ||
|} | |} | ||
Revision as of 12:05, 4 December 2015
This page can now be filled with content.
Model Formulation
Differential equation system:
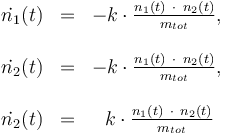
Reaction velocity constant:
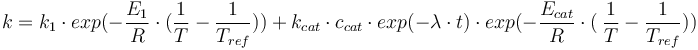
Total mass:
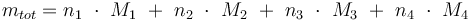
Temperature in Kelvin:
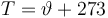
| Name | Symbol | Initial value (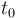 ) )
|
| Molar number 1 | 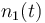
|
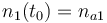
|
| Molar number 2 | 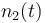
|
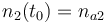
|
| Molar number 3 | 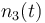
|
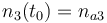
|
| Name | Symbol | Value |
| Steric factor | 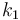
|
X |
| Steric factor | 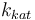
|
X |
| Activation energie | 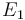
|
X |
| Activation energie | 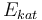
|
X |
| Catalyst deactivation coefficient | 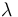
|
X |
| Name | Symbol | Value | Interval |
| Initial molar number 1 | 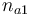
|
[0.4,9.0] | |
| Initial molar number 2 | 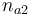
|
[0.4,9.0] | |
| Initial molar number 3 | 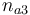
|
[0.4,9.0] | |
| Concentration of the catalyst | 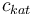
|
[0.0,6.0] | |
| Initial molar number 1 | 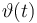
|
[20.0,100.0] |