Difference between revisions of "LinearMetabolic"
m (Tsiantis reference) |
m (Introductory text) |
||
| Line 9: | Line 9: | ||
--> | --> | ||
| − | + | The '''Linear Metabolic problem''' is a generalized inverse optimal control problem formulated and investigated in <bib id="Tsiantis2018" />. It tries to identify an a priori unknown objective function from data. | |
| − | The | + | The problem is a generalization of the one studied by de Hijas-Liste et al. (2014), where it was considered as a standard optimal control problem (OCP). Here, we take the solution reference of the inner problem as the multi-objective OCP described in de Hijas-Liste et al. (2014), selecting a specific point of the resulting Pareto front. This case study is interesting, because it includes path constraints on the states and the inputs. |
| − | + | A 3-step linear metabolic pathway with mass action kinetics is considered. The differential states <math>x</math> are metabolite concentrations, the time-dependent control functions <math>u</math> are enzyme concentrations, and the model parameters <math>p</math> are kinetic parameters in mass action expressions. Candidate objective functionals <math>\phi</math> are the final time and the time-integral of the intermediate metabolite concentrations. The measurement function is a map to the values of the differential states and comprises measurements of metabolite concentrations. The differential equations are assumed to be fully known as standard mass action kinetics. An inequality path constraint is present (but potentially unknown in such settings) on the inner level and critical from a biological point of view: limitations due to molecular crowding impose an upper bound on the maximum total concentration of enzymes (controls) at any given time. Boundary conditions are fixed initial and terminal values of the metabolite concentrations. | |
== Mathematical formulation == | == Mathematical formulation == | ||
| − | The | + | Under construction... |
| + | |||
| + | The gIOC is given by | ||
<p> | <p> | ||
Revision as of 14:48, 19 October 2023
| LinearMetabolic | |
|---|---|
| State dimension: | 1 |
| Differential states: | 4 |
| Discrete control functions: | 1 |
| Path constraints: | 2 |
| Interior point inequalities: | 2 |
| Interior point equalities: | 5 |
The Linear Metabolic problem is a generalized inverse optimal control problem formulated and investigated in [Tsiantis2018]Author: Tsiantis, Nikolaos; Balsa-Canto, Eva; Banga, Julio R
Journal: Bioinformatics
Number: 14
Pages: 2433-2440
Title: Optimality and identification of dynamic models in systems biology: an inverse optimal control framework
Volume: 34
Year: 2018 . It tries to identify an a priori unknown objective function from data.
. It tries to identify an a priori unknown objective function from data.
The problem is a generalization of the one studied by de Hijas-Liste et al. (2014), where it was considered as a standard optimal control problem (OCP). Here, we take the solution reference of the inner problem as the multi-objective OCP described in de Hijas-Liste et al. (2014), selecting a specific point of the resulting Pareto front. This case study is interesting, because it includes path constraints on the states and the inputs.
A 3-step linear metabolic pathway with mass action kinetics is considered. The differential states 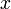 are metabolite concentrations, the time-dependent control functions
are metabolite concentrations, the time-dependent control functions 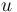 are enzyme concentrations, and the model parameters
are enzyme concentrations, and the model parameters 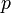 are kinetic parameters in mass action expressions. Candidate objective functionals
are kinetic parameters in mass action expressions. Candidate objective functionals 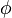 are the final time and the time-integral of the intermediate metabolite concentrations. The measurement function is a map to the values of the differential states and comprises measurements of metabolite concentrations. The differential equations are assumed to be fully known as standard mass action kinetics. An inequality path constraint is present (but potentially unknown in such settings) on the inner level and critical from a biological point of view: limitations due to molecular crowding impose an upper bound on the maximum total concentration of enzymes (controls) at any given time. Boundary conditions are fixed initial and terminal values of the metabolite concentrations.
are the final time and the time-integral of the intermediate metabolite concentrations. The measurement function is a map to the values of the differential states and comprises measurements of metabolite concentrations. The differential equations are assumed to be fully known as standard mass action kinetics. An inequality path constraint is present (but potentially unknown in such settings) on the inner level and critical from a biological point of view: limitations due to molecular crowding impose an upper bound on the maximum total concentration of enzymes (controls) at any given time. Boundary conditions are fixed initial and terminal values of the metabolite concentrations.
Contents
Mathematical formulation
Under construction...
The gIOC is given by
![\begin{array}{llclr}
\displaystyle \min_{x, u} & x_3(t_f) \\[1.5ex]
\mbox{s.t.}
& \dot{x}_0 & = & (V_{alim} u - R_m x_0 - K_m x_1) / L_m, \\
& \dot{x}_1 & = & \frac{K_r^2}{Mr^2} (K_m x_0 - \frac{r}{K_r} ( M g K_f + \frac{1}{2} \rho S C_x \frac{r^2}{K_r^2} x_1^2)), \\
& \dot{x}_2 & = & \frac{r}{K_r} x_1, \\
& \dot{x}_3 & = & V_{alim} u x_0 + R_{bat} x_0^2, \\[1.5ex]
& x(t_0) &=& (0,0,0,0)^T, \\
& x(t_f) & \in & \mathcal{T} \subseteq \mathbb{R}^4,\\
& x_0(t) & \in & [-i_{max}, i_{max}], \\
& u(t) &\in& \{-1, 1\}.
\end{array}](https://mintoc.de/images/math/f/8/1/f81a52adee4116324d4656c5e4256abb.png)
Here the four differential states stand for the electrical current (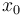 ), the angular velocity (
), the angular velocity (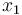 ), the position of the car (
), the position of the car (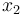 ), and the consumed energy (
), and the consumed energy (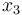 ). The objective function
). The objective function 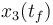 is just a reformulation of the Lagrange-type objective function tracking the used energy over time.
is just a reformulation of the Lagrange-type objective function tracking the used energy over time.
Parameters
These fixed values are used within the model.
| Name | Symbol | Value | Unit |
| Coefficient of reduction | 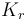
|
10 | [-] |
| Air density | 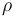
|
1.293 | 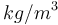
|
| Aerodynamic coefficient | 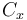
|
0.4 | [-] |
| Area in the front of the vehicle | 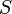
|
2 | 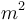
|
| Radius of the wheel | 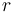
|
0.33 | 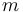
|
| Constant representing the friction of the wheels on the road | 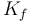
|
0.03 | [-] |
| Coefficient of the motor torque | 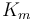
|
0.27 | [-] |
| Inductor resistance | 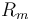
|
0.03 | 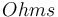
|
| inductance of the rotor | 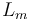
|
0.05 | [-] |
| Mass | 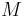
|
250 | 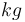
|
| Gravity constant | 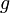
|
9,81 | [-] |
| Battery voltage | 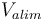
|
150 | 
|
| Resistance of the battery | 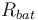
|
0.05 | 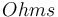
|
Reference Solutions
We look at the particular instance of our problem with 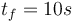 and target set
and target set 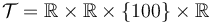 , in which the car needs to cover 100m in 10s.
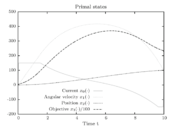
Figure 1 shows a plot of the differential states of the optimal trajectory of the relaxed problem (i.e.
, in which the car needs to cover 100m in 10s.
Figure 1 shows a plot of the differential states of the optimal trajectory of the relaxed problem (i.e. ![u \in [-1,1]](https://mintoc.de/images/math/7/7/e/77ebca4c196d281ab127d484baf6bd92.png) instead of
instead of 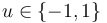 ) for
) for 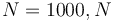 being the number of time discretization points. The current
being the number of time discretization points. The current 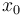 increases to its maximal value of 150A, stays there for a certain time, decreases on its minimal value of -150A, stays on this value and eventually increases slightly. This behavior corresponds to the different arcs bang, path-constrained, singular, path-constrained, bang and can be observed also in Figure 2. It shows the corresponding switching function and the optimal control. Note that the plots show data from the solution with the indirect approach.
increases to its maximal value of 150A, stays there for a certain time, decreases on its minimal value of -150A, stays on this value and eventually increases slightly. This behavior corresponds to the different arcs bang, path-constrained, singular, path-constrained, bang and can be observed also in Figure 2. It shows the corresponding switching function and the optimal control. Note that the plots show data from the solution with the indirect approach.
Applying the sum up rounding strategy results in an integer-feasible chattering solution.
The resulting primal states are shown in Figure 3. One observes the high-frequency zig-zagging of the current 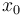 that results from the fast switches in the control.
that results from the fast switches in the control.
The direct and indirect approaches are local optimization techniques and only provide upper bounds for the relaxed problem and hence for the original problem. Here the indirect solution of the relaxed problem gives us a bound of 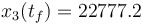 .
.
- Reference solution plots
Source Code
Model descriptions are available in
Variants
There are several alternative formulations and variants of the above problem, in particular
- fixed final velocity,
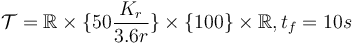
- bounded velocity,
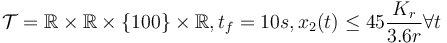
- fixed final velocity, bounded velocity, longer time horizon,
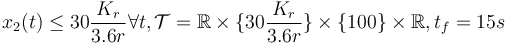 .
.
References
| [Tsiantis2018] | Tsiantis, Nikolaos; Balsa-Canto, Eva; Banga, Julio R (2018): Optimality and identification of dynamic models in systems biology: an inverse optimal control framework. Bioinformatics, 34, 2433-2440 |  |
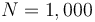 .
.

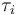 where transitions between different kinds of arcs occur.
where transitions between different kinds of arcs occur.

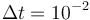 .
.